Answering questions (and dispelling myths) about virtual clinical research models.
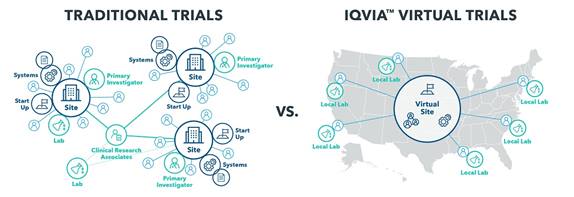
Virtual trials promise to transform the way clinical research is conducted. This model delivers multiple benefits including access to larger patient populations, easier recruiting, more and better data collection, lower levels of attrition, and ultimately lower costs.
Yet because this model is still relatively new, many industry professionals are uncertain about how it works, where it’s appropriate, and what it means for site staff. To address these concerns, we’ve answered the most common questions when it comes to virtual trials.
- Do patients ever see a principal investigator? Yes. Most patients in virtual trials will have scheduled visits via telemedicine with PIs and other clinicians over the course of the trial. The ease of use of live face-to-face virtual meeting tools means PIs can meet with patients more frequently than they might in a traditional trial, and can quickly connect with patients if they have additional questions or are dealing with side effects.
- What if the patient needs help with a treatment? Virtual trial leaders can deploy nurses, phlebotomists, or other care providers directly to the patient’s home to deliver a treatment the patients can’t or won’t complete on their own. If necessary, the PI can join via telemedicine to oversee the process.
- What if a trial only needs a few on-site visits, can it still be virtual? Perhaps. We’ve seen hybrid models that require a few initial site visits, followed by a predominantly virtual experience.
- How does a virtual trial reach a broader patient population? Most traditional trial participants live within 50 miles of a site due to travel and time limitations, which reduces the pool of patients who can participate in any study or site location. Because virtual trials require no site visits, it eliminates this barrier, allowing PIs to recruit from a much broader geography of patients, and oversee trials for an entire state or region.
- Can any trial be virtual? No. Thanks to advances in connected devices, patient-reported-outcome platforms, and in-home treatment options, this model is applicable for a surprisingly broad number of treatment areas, including central nervous system (CNS), dermatology, cardiovascular, respiratory, endocrinology, and long-term extended trials in oncology. However it’s not right for every study. If a clinical trial requires complex treatment procedures, in-patient care or observation, or involves acutely ill patients it is not appropriate.
- Is it more expensive than traditional trials? No, in fact it’s less expensive because a single PI can oversee a larger number of patients, reducing the cost of managing multiple sites and staff. And because the virtual model lessens the burden of participation on patients and caregivers, recruiting is accelerated, which shortens trial duration and speeds results. All of this contributes to a more cost-effective trial environment.
- Won’t this model reduce opportunities for sites to participate in clinical studies? Yes and no. Virtual trials do require fewer PIs and less time on the part of the site staff, but many sites see this as an opportunity. Because virtual trials are less time-intensive, sites can oversee more trials simultaneously, and free their staff to focus on more complex in-house patient visits. It’s also a great opportunity for PIs who’d like to work fewer hours, with lower financial and administrative burdens, but still want to stay involved in research.
- Do site staff have to provide tech support? No. The virtual trial model includes a “site concierge” who onboards virtual patients to the site platform and patient portals, answers questions about wearable devices, and provides support for technology issues that arise. This ensures busy healthcare professionals can focus on clinical tasks.
- Won’t data quality suffer? No. In fact we have found the opposite to be true. All of the data in virtual trials is captured digitally, much of it via wearable or implantable devices. This eliminates the risk of transcription error, and increases the quantity and consistency of data reported. Many of these devices capture thousands of data points throughout the day and transmit them in real time to the research database, giving PIs a more robust picture of the patient’s health and response to treatments.
- Is it safe? Yes. The technology-enabled nature of virtual trials makes them as safe as, and in some cases, safer than traditional trial environments. The use of telemedicine means patients often have more interaction with PIs, and can schedule additional calls if they have concerns or experience side effects. The connected devices used in these trials also include alerts, reminders, and warning systems if patients fail to take their medicine, or experience a negative reaction, which increases safety and protocol adherence.
- Do regulators approve of this model? Regulators in every country have their own rules and opinions, but we are seeing a steady move toward embracing virtual trials. There are several virtual trials already under way in the US with regulatory approval, and regulators across the EU are currently examining these models for use beginning in 2019. Overall, our experience has been that regulators are increasingly interested in the use of digital technology and connected devices to improve clinical research. As long as the protocols are sound and adhered to, most regulators see virtual trials as an opportunity. Companies that are uncertain about whether virtual trial results will be accepted, should discuss their plans with regulators before moving forward.
Virtual trials offer huge potential to ease recruiting and increase patient engagement while lowering the overall cost of conducting these studies. We hope this clears up any misperceptions you have about virtual trials.
IQVIA Virtual Trials is uniquely qualified to orchestrate the unique complexity of patient-centered virtual trials to accelerate the path to approval. Click here to learn more.