Build on our experience of more than 245 rare disease studies in 96 countries to fulfill your promise of hope to millions around the world.
With new legislative initiatives from regulatory bodies in a number of healthcare markets, coupled with a high-unmet medical need, pharmaceutical companies are continuing to invest in the development of orphan drugs. This article provides an analysis of key 2019 orphan drug approvals, using IQVIA Pipeline Intelligence data.
Introduction
Rare diseases, for which orphan drugs are indicated to treat, are characterized by a low frequency of occurrence in the general population of a geographical region. There are over 8000 described rare diseases, of which only 340 conditions currently have treatment options1. Some of these diseases, such as progeria, occur in 1 in every 4 million births, while others may be comparatively more common, such as multiple sclerosis, cystic fibrosis and Duchenne muscular dystrophy. The prevalence of these diseases varies between populations, making an indication rare for one region but common for another. The effects of these rare diseases range from premature mortality to severe disability due to a lack of efficacious treatment options. Growing understanding of numerous aspects of these diseases, along with improving government policies, are expanding the framework for research, development and market penetration through the introduction of novel and renovated therapies.
2019 Orphan Drug Approval Overview
The improvement in government policies over the years has been mirrored by an increase in the development activities for candidates addressing rare diseases; with less than 10 approved rare disease therapies on the market in the 1970s increasing to more than 830 US FDA approved orphan therapies as of 20192. New treatment options have been brought to market through the launch of either new molecular entities or through the renovation of older, established therapies.
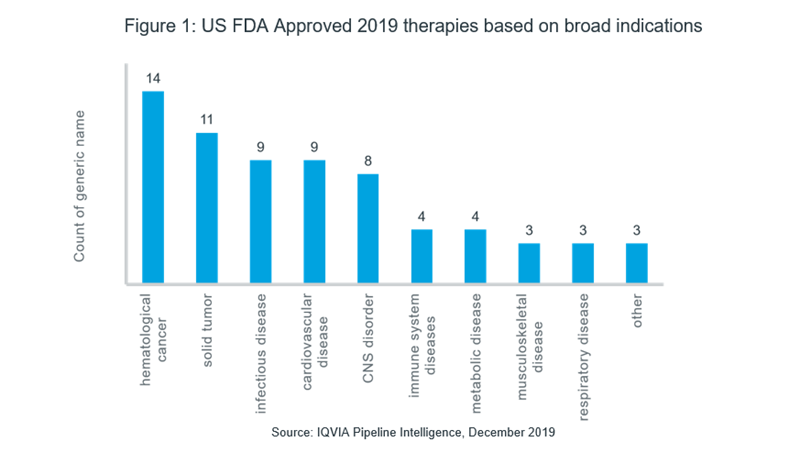
As shown in Figure 1, the US FDA granted approval to more than 65 therapies2 in various rare disease therapeutic areas with the most approvals seen in cancer. Among them, hematological cancers have seen the maximum approvals, with 14 therapies for a range of indications including; multiple myeloma, myelofibrosis, acute lymphoid leukemia and various forms of lymphomas approved in 2019. This is followed by 11 registered therapies for solid tumors, including small cell lung cancer, non-small cell lung cancer, ovarian cancer, hepatocellular cancer, esophageal cancer, neuroendocrine tumor, melanoma and NTRK fusion-positive tumors. Furthermore, 9 therapies were approved for infectious diseases, including 2 therapies for tuberculosis, 3 for hepatitis C virus infection, 2 for human immunodeficiency virus infection and a therapy for fascioliasis. With 8 therapy approvals in the USA for disorders of the central nervous system, including Lambert-Eaton myasthenic syndrome, neuromyelitis optica, spinal muscular atrophy, amyotrophic lateral sclerosis and narcolepsy, the needs of another major therapeutic area have to some extent, been catered to. Additionally, 2 biologic therapies have been approved for Churg-Strauss Syndrome, 1 for graft versus host disease and 1 for systemic sclerosis in the field of autoimmune diseases.
Conclusion
With increasing focus from governments and regulatory bodies, coupled with a high-unmet medical need, pharmaceutical companies will continue to invest in the development of orphan drugs. In particular, ‘Big Pharma’ will continue to explore this field, through its own organic development and through the acquisition of small start-ups as well as established players. Additionally, the recent launch of a one-time adeno-associated virus mediated human RPE65 gene therapy for Leber congenital amaurosis, clearly establishes the will of big pharma to explore the orphan drug space, despite the financial and research headwinds.
References
- Ekins, S. Industrializing rare disease therapy discovery and development. Nature Biotechnology 2017; 35(2), 117–118.
- US FDA Orphan Drug list access portal. Available at: http://bit.ly/34xkdDg
- Melnikova, I. Rare diseases and orphan drugs. Nature Reviews Drug Discovery, 2012;11(4), 267–268.
To find out more about IQVIA Pipeline Intelligence is, please visit
https://www.iqvia.com/solutions/commercialization/brand-strategy-and-management/product-pipeline
Related solutions
Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.

























