What is claims data?
Any data that comes from processing a healthcare claim is known as claims data. Claims data can be sourced from health plans (commonly known as a closed claims database) or from practice management systems/clearinghouses (open claims database). Though claims are primarily processed for payment purposes, the information collected from claims are used for secondary research in healthcare to understand patients’ interactions with the healthcare system and infer patients’ and prescribers’ behavior.
What do we mean by “closed” claims data?
A claims database such as IQVIA’s PharMetrics® Plus, is an example of a multi-payer, closed claims data source. This is a closed database of adjudicated medical and pharmacy claims in the U.S. and contains patient enrollment information. IQVIA’s PharMetrics Plus brings data from long-standing partnerships, such as Blue Health Intelligence® (BHI®) and continues to grow with new relationships.
“Since 2012, IQVIA and BHI continue to partner together to help provide the industry with a gold standard closed claim database along with IQVIA’s expertise in data science, epidemiology, and health economics. Our relationship with BHI strengthens the PharMetrics Plus offering,” said Lisa Morris, Vice President & General Manager, Real World Solutions, IQVIA.
Closed claims databases such as PharMetrics Plus, that contain fully adjudicated cost and patient enrollment information, have been considered the gold standard to analyze a complete patient journey. The beauty of this closed database is that it has a month-by-month indication that a patient is enrolled with medical benefits, pharmacy benefits, or both.
Here are some common ways closed claims data is used
With the patient enrollment and cost information in PharMetrics Plus, researchers can support key analyses when examining healthcare cost and resource utilization, disease burden, and a patient’s journey across healthcare settings.
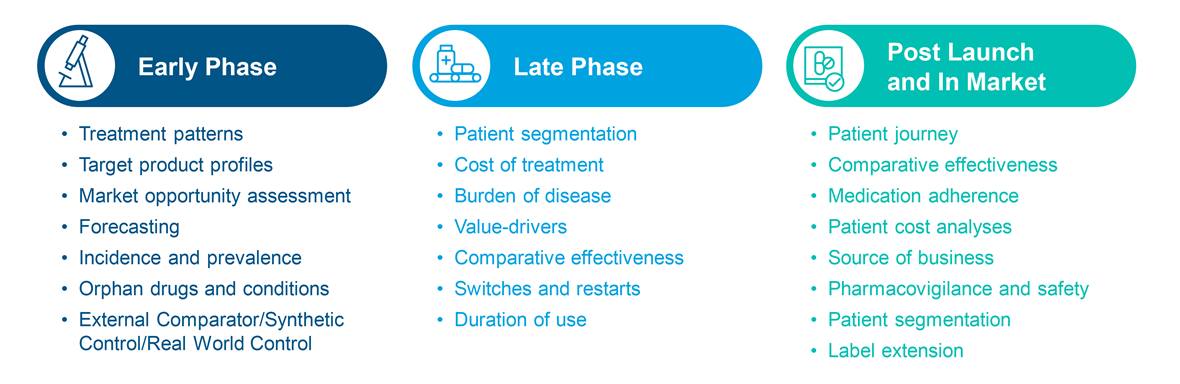
How else can closed claims data be used?
1. Layer it with open claims
IQVIA’s Longitudinal Prescription Data (LRx) or Medical Claims Data (Dx) are examples of open source databases which are sourced from pharmacies, practice management systems, and clearinghouses. With a 1-3 week lag and access to a huge volume of claims, the open source claims databases provide quick insight into
- Launch and market uptake
- Managed market impact analysis
- Physician targeting and messaging
- Source of business
- Diagnosis allocation
- Patient journey
Since these open claims data are not sourced from health plans, open claims can capture cash payment information and can be used to analyze such transactions not covered by plans. When closed claims data is layered with open claims, we can have visibility into cash transactions on top of the allowed and paid amount obtained from the closed claims.
For example, a Health Resource Utilization (HRU) study driven by PharMetrics Plus, can layer on IQVIA’s longitudinal prescription data (LRx) to provide insight into prescriptions where the patient was paying cash, typically seen for generic drugs.
In one study1, data from 965,785 patients with statin claims in PharMetrics Plus were analyzed. The study found that 20.1% of the study cohort had ≥ 1 missing statin claim post-index. The most common reasons for missing data were payment by an alternate third-party program (66.3%) and use of cash, coupon, or discount cards (18.7%).
2. Bring in clinical metrics
Clinical metrics are incredibly useful in assessing the cost of treatment specific to sub-markets, in obtaining better patient stratification by disease severity or vitals, and in analyzing treatment start triggers based on lab values. For example, segmenting the patients of interest through the lens of their vitals measurements (e.g. by height/weight/BMI, by systolic/diastolic blood pressure, by oxygen saturation, etc.) could add yet another dimension to the insights. It could take the analysis a level deeper, getting closer to the “why” behind differences observed in the data.
Since claims data lack clinical metrics, leveraging clinical metrics can answer important questions such as
- Do patients with a certain level of disease severity react differently to some medications versus others?
- Does staging for cancer patients play a role in initiation of therapy/moving to a second line?
- How large is the treated but still uncontrolled patient segment?
- Are providers waiting too long to treat based on lab results?
- What is the burden of disease by severity?
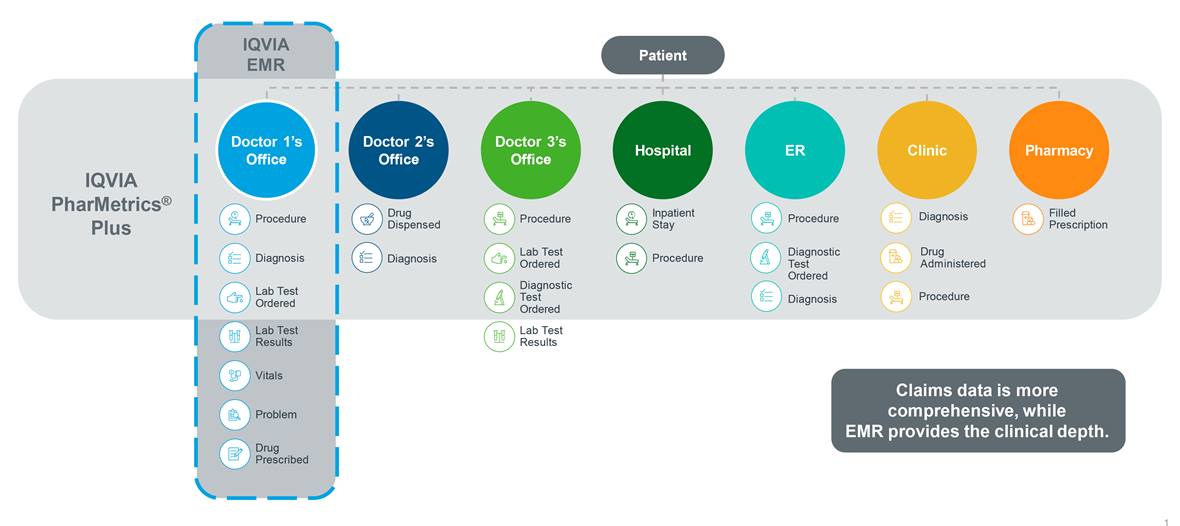
Typically, claims data would not be used to gain insights into a patient’s encounter with a physician or to understand a patient’s prescription filling behavior. However, when it comes to providing detailed clinical information required to measure outcomes of interest that are focused on disease morbidity or severity, claims data alone might not be sufficient for your research needs. That’s why researchers across the globe are combining claims data with electronic medical records (EMR), laboratory results, and patient reported outcomes (PRO) data to obtain new and more detailed insights into disease severity, progression, morbidity, and mortality. These datasets offer a unique perspective to add to the patient experience, visible only by using closed claims data in the research.
For example, robust studies could be designed with cancer-related clinical metrics (e.g. staging, tumor grades) when PharMetrics Plus is linked to IQVIA Oncology EMR data. You could link patients identified in PharMetrics Plus to IQVIA Ambulatory EMR for more in-depth view of their clinical metrics for your analysis.
Claims and EMR data provide different views of a patient’s encounter with the healthcare system.
IQVIA’s experts maximize value from closed claims data
IQVIA has been performing research with closed claims data ever since health plan data were considered the gold standard for secondary data. PharMetrics Plus has been cited in many award-winning publications that have received Gold and Silver Ribbons at Academy of Managed Care Pharmacy (AMCP). Additionally, it has been used in many publications at International Society of Pharmacoeconomics and Outcomes Research (ISPOR) for more than 10 years in collaboration with leading industry partners.
IQVIA experts access large amounts of data from various sources, and have layered them with closed claims data and published the findings in many peer reviewed journals and scientific meetings/conferences. Examples of some recent publications are noted at the end of this blog.
Conclusion
Health plan claims (closed claims) data sources have served as the foundation for retrospective data analysis by providing powerful insights into patients’ diagnosis, treatment, medication use, healthcare cost and resource utilization for years. In the last decade, combining closed claims data with other types of data via linking has increased and has enabled researchers to glean even more powerful insights than using claims alone.
IQVIA guides customers in their efforts to identify, obtain, and layer relevant data sources to arrive at the optimal study cohort with optimal data history, and aids in conducting a wide variety of studies.
With decades of experience in the human data ecosystem, IQVIA can provide operation-grade data sources for customers to smoothly move from insights to activation. Beyond that vast volume of reliable and readily available data, IQVIA Connected Intelligence™ can you help you to discover new insights, drive smarter decisions, and unleash new opportunities to meet and exceed business and research needs.
Examples of Some Recent Publications
Gold Ribbon at AMCP, 2021: Norregaard C., Hull M., Shah D., Cohen J., Carroll B., DeKoven M., He J., Sullivan E. Healthcare burden of patients newly diagnosed with moderate to severe non-advanced systemic mastocytosis using a real-world database of US health plan members.
Silver Ribbon at AMCP, 2021: Postma M., Divino V., Pelton S., Mould-Quevedo J., Anupindi V., DeKoven M., Krishnarajah G., et al. Economic Assessment of the Cell-Based Influenza Vaccine versus the Standard Egg-Based Quadrivalent Influenza Vaccine in the U.S.: A Real-World Evidence Estimation of Healthcare Costs for the 2018-19 Influenza Season. AMCP 2021 Virtual.
Finalist at ISPOR, 2019: Divino V., Schranz J., Shah H., Jiang M., DeKoven M., Zilberberg M. Economic Outcomes of Patients with Community-Acquired Pneumonia (CAP) Hospitalizations in The US: A Retrospective Cohort Study, 2012-2017. PIN50, ISPOR 2019, New Orleans, LA, USA.

Fact Sheet: IQVIA PharMetrics® Plus
Access comprehensive real world data on commercially insured patients in the U.S. with a view into their diagnoses, treatments, and associated costs.








