-
Americas
-
Asia & Oceania
-
A-I
J-Z

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more -
Middle East & Africa

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more
Regions
-
Americas
-
Asia & Oceania
-
Europe
-
Middle East & Africa
-
Americas
-
Asia & Oceania
-
Europe
Europe
- Adriatic
- Belgium
- Bulgaria
- Czech Republic
- Deutschland
- España
- France
- Greece
- Hungary
- Ireland
- Israel
- Italia

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more -
Middle East & Africa

EMEA Thought Leadership
Developing IQVIA’s positions on key trends in the pharma and life sciences industries, with a focus on EMEA.
Learn more
SOLUTIONS
-
Research & Development
-
Real World Evidence
-
Commercialization
-
Safety & Regulatory Compliance
-
Technologies
LIFE SCIENCE SEGMENTS
HEALTHCARE SEGMENTS
- Information Partner Services
- Financial Institutions
- Public Health and Government
- Patient Associations
- Payers
- Providers
THERAPEUTIC AREAS
- Cardiovascular
- Cell and Gene Therapy
- Central Nervous System
- GI & Hepatology
- Infectious Diseases and Vaccines
- Oncology
- Pediatrics
- Rare Diseases
- View All

Impacting People's Lives
"We strive to help improve outcomes and create a healthier, more sustainable world for people everywhere.
LEARN MORE
Harness the power to transform clinical development
Reimagine clinical development by intelligently connecting data, technology, and analytics to optimize your trials. The result? Faster decision making and reduced risk so you can deliver life-changing therapies faster.
Research & Development OverviewResearch & Development Quick Links

Real World Evidence. Real Confidence. Real Results.
Generate and disseminate evidence that answers crucial clinical, regulatory and commercial questions, enabling you to drive smarter decisions and meet your stakeholder needs with confidence.
REAL WORLD EVIDENCE OVERVIEWReal World Evidence Quick Links

See markets more clearly. Opportunities more often.
Elevate commercial models with precision and speed using AI-driven analytics and technology that illuminate hidden insights in data.
COMMERCIALIZATION OVERVIEWCommercialization Quick Links

Service driven. Tech-enabled. Integrated compliance.
Orchestrate your success across the complete compliance lifecycle with best-in-class services and solutions for safety, regulatory, quality and medical information.
COMPLIANCE OVERVIEWSafety & Regulatory Compliance Quick Links

Intelligence that transforms life sciences end-to-end.
When your destination is a healthier world, making intelligent connections between data, technology, and services is your roadmap.
TECHNOLOGIES OVERVIEWTechnology Quick Links
CLINICAL PRODUCTS
COMMERCIAL PRODUCTS
COMPLIANCE, SAFETY, REG PRODUCTS
BLOGS, WHITE PAPERS & CASE STUDIES
Explore our library of insights, thought leadership, and the latest topics & trends in healthcare.
DISCOVER INSIGHTSTHE IQVIA INSTITUTE
An in-depth exploration of the global healthcare ecosystem with timely research, insightful analysis, and scientific expertise.
SEE LATEST REPORTSFEATURED INNOVATIONS
-
IQVIA Connected Intelligence™
-
IQVIA Healthcare-grade AI™
-
Human Data Science Cloud
-
IQVIA Innovation Hub
-
Decentralized Trials
-
Patient Experience powered by Apple
WHO WE ARE
- Our Story
- Our Impact
- Commitment to Public Health
- Code of Conduct
- Environmental Social Governance
- Privacy
- Executive Team
NEWS & RESOURCES

Unlock your potential to drive healthcare forward
By making intelligent connections between your needs, our capabilities, and the healthcare ecosystem, we can help you be more agile, accelerate results, and improve patient outcomes.
LEARN MORE
IQVIA AI is Healthcare-grade AI
Building on a rich history of developing AI for healthcare, IQVIA AI connects the right data, technology, and expertise to address the unique needs of healthcare. It's what we call Healthcare-grade AI.
LEARN MORE
Your healthcare data deserves more than just a cloud.
The IQVIA Human Data Science Cloud is our unique capability designed to enable healthcare-grade analytics, tools, and data management solutions to deliver fit-for-purpose global data at scale.
LEARN MORE
Innovations make an impact when bold ideas meet powerful partnerships
The IQVIA Innovation Hub connects start-ups with the extensive IQVIA network of assets, resources, clients, and partners. Together, we can help lead the future of healthcare with the extensive IQVIA network of assets, resources, clients, and partners.
LEARN MORE
Proven, faster DCT solutions
IQVIA Decentralized Trials deliver purpose-built clinical services and technologies that engage the right patients wherever they are. Our hybrid and fully virtual solutions have been used more than any others.
LEARN MORE
IQVIA Patient Experience Solutions powered by Apple
Empowering patients to personalize their healthcare and connecting them to caregivers has the potential to change the care delivery paradigm. IQVIA and Apple are collaborating to bring this exciting future of personalized care directly to devices patients already have and use.
LEARN MOREWORKING AT IQVIA
Our mission is to accelerate innovation for a healthier world. Together, we can solve customer challenges and improve patient lives.
LEARN MORELIFE AT IQVIA
Careers, culture and everything in between. Find out what’s going on right here, right now.
LEARN MORE
WE’RE HIRING
"Improving human health requires brave thinkers who are willing to explore new ideas and build on successes. Unleash your potential with us.
SEARCH JOBS- Blogs
- Sickle Cell R&D Success Stories
Leveraging knowledge, operational strategies, data, and relationships to accelerate recruitment while maintaining quality and timely delivery
IQVIA has an extended history of contributions to sickle cell disease therapeutic development efforts around the world. Many of the studies have been successful, with accelerated timelines and high quality results, despite facing and overcoming roadblocks and challenges.
Key Contributions from the Middle East and Africa: Therapeutic and regional knowledge and operational strategies to secure quality and timely deliveryOne study in adult patients with sickle cell disease was conducted across 8 countries across North America, Europe, the Middle East, and Northern and sub-Saharan Africa. Involvement of these regions was critical to the success of the execution strategy; however, the sponsor was naïve to working in the Middle East and Africa and had some reservations. The study was further challenged by IP and device importation regulatory changes during start-up, central lab and sample oversight logistical issues, concerns around protocol compliance of young adults, and validation of a patient reported outcomes tool in new languages.
With our therapeutic and regional knowledge, complimented by operational strategies designed to address the study-specific challenges, we were able to secure quality and timely delivery for the study. IQVIA’s expertise helped the sponsor navigate the local and regional complexities they faced. IQVIA engaged passionate KOLs and team medics in communication with and motivation of sites. Robust feasibility and validation of recruitment projections was closely managed with real time site input and historical experience. Flexibility was maximized to support competitive recruitment (e.g., equipment needs and IP transport to patients in rural areas). Effective risk management enabled adequate site support and data quality at high-enrolling centers and proactive planning for database lock based upon recruitment patterns and volume and impact on data cleaning efforts with internal and site staff.
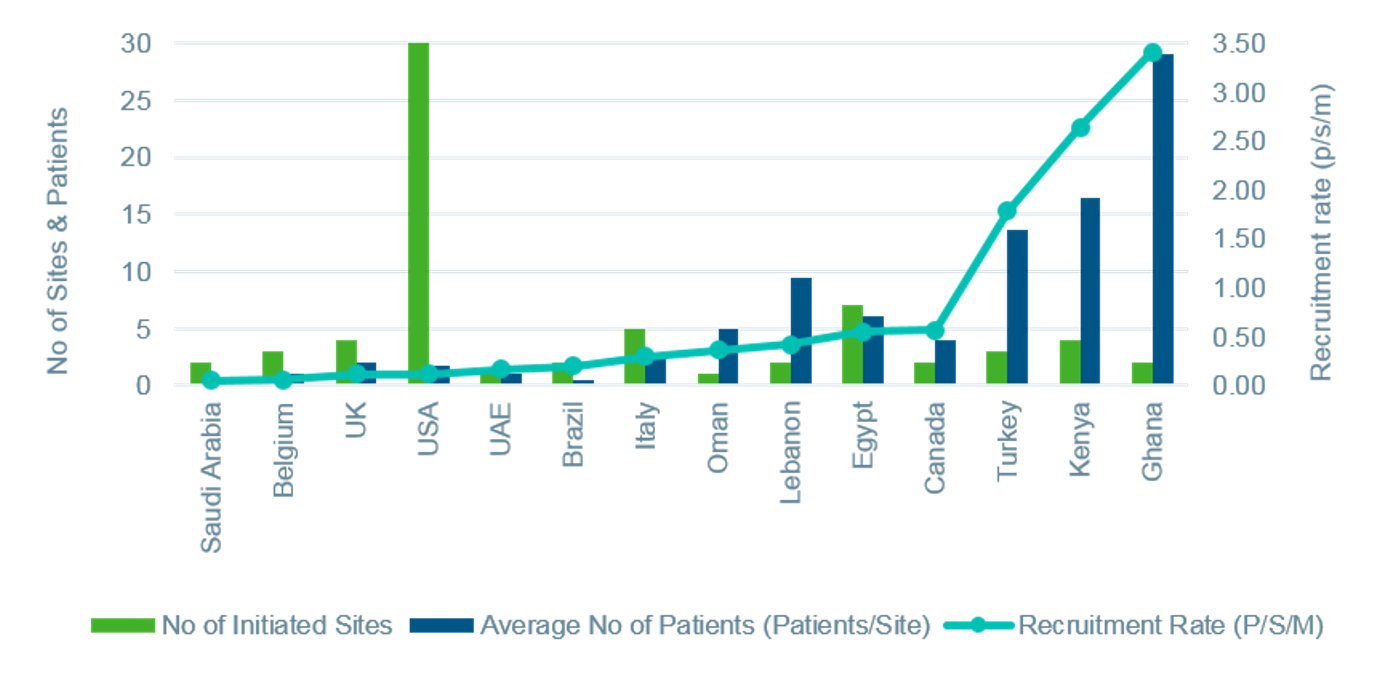
As a result of IQVIA’s expertise and effective execution, the first subject was randomized approximately one month ahead of schedule. Involvement and engagement of countries with high prevalence of sickle cell disease was key to the successful delivery strategy. Increasing sponsor comfort and effectively managing regional nuances ensured the phenomenal overall recruitment productivity of the study with low screen failure rates. Ultimately, the final CSR was delivered on time and per the original target.
Another example further demonstrates IQVIA’s insight and understanding of successful sickle cell study delivery. In a treatment study recruiting adult and pediatric patients, partnering with sites in the Middle East and Africa was essential to satisfying recruitment expectations. However, longer start-up timelines in some countries as well as malaria co-infection among the SCD population increased the degree of complexity, and an agreement had to be negotiated on several unique budget requirements (e.g., transportation and courier costs, vehicles to deliver IP to patients in rural areas, solar chargers and other protocol-required equipment).
IQVIA leveraged our relationships and experience to engage the right expert Middle East and Africa sites. Slower start-up in this region was balanced against higher recruitment and lower drop-out rates compared to Europe and North America. Sites in the Middle East and Africa also demonstrated the highest retention rates, preserving critical endpoint data. By understanding and identifying the capability and needs of these key sites, IQVIA helped secure the success of this important investigation.
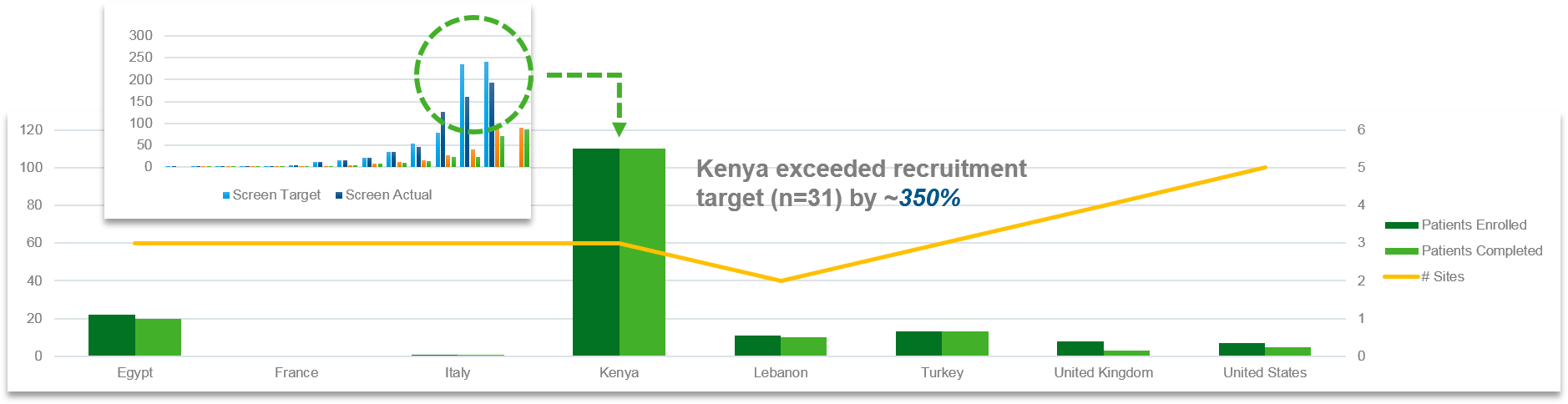
Success in Latin America: Leveraging data and relationships to accelerate recruitment while maintaining qualityIQVIA also conducted a study recently, targeting 30 adult patients with sickle cell disease for an investigational treatment. The study was conducted over 12 months across several sites in Latin America including Honduras, the Dominican Republic, Panama, and Columbia. To reach the right patients, research naïve sites had to be engaged. Furthermore, start-up was slower than anticipated, resulting in an unexpected 5-month delay.
These complications understandably exacerbated stakeholder anxiety relative to successful completion of the study. To overcome the challenges facing the study, IQVIA implemented an enhanced communication strategy with close site contact and follow-up to support questions and quickly pre-identify and validate screening and enrollment expectations weekly. To better engage sites, communications were handled in English and Spanish, and site concerns were rapidly escalated and quickly addressed. Routine progress updates and newsletters were used to foster not only an environment of healthy competition among sites, but solidarity and celebration of joint success in achieving the study’s overarching goals as well.
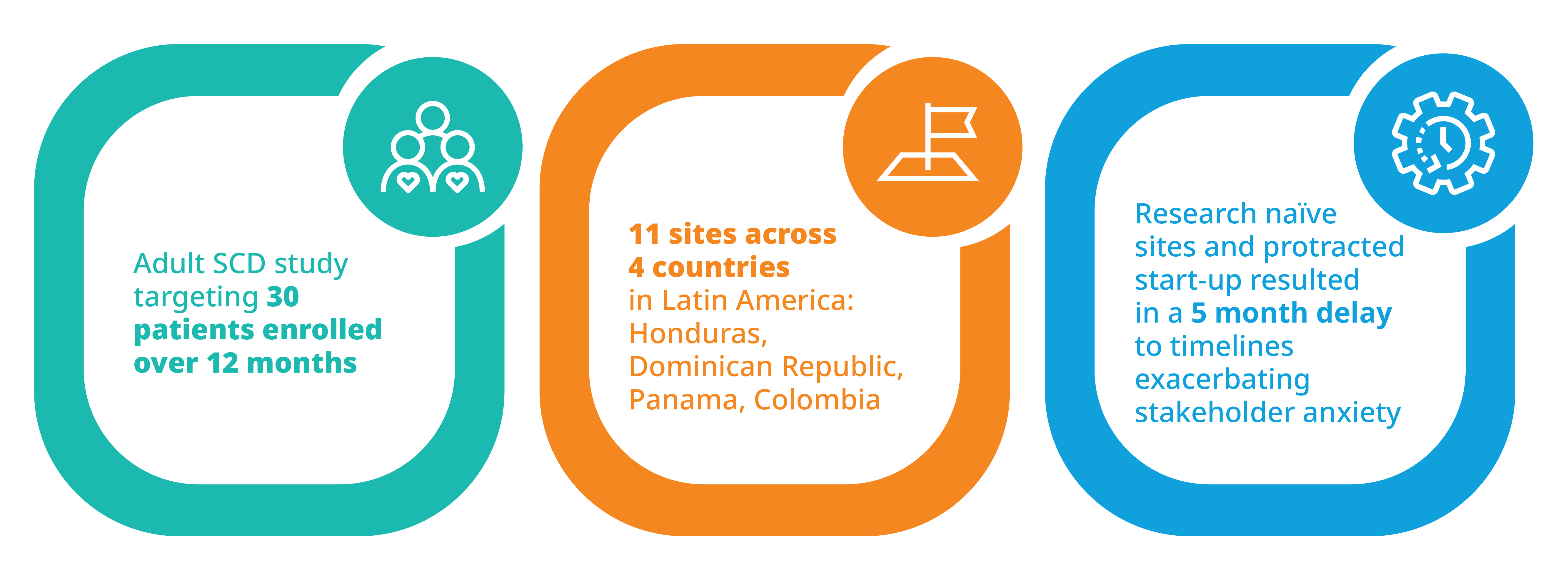
In the end, IQVIA’s efforts, through its close local relationships and its data capabilities, resulted in the recruitment of 34 patients over six months, with a net acceleration of one month relative to the original timeline. Moreover, the screen failure rate was significantly lower than anticipated (14% reduction).











