IQVIA is using vast quantities of data in powerful new ways. See how we can help you tap into information from past trials, patient reported outcomes and other sources to accelerate your research.




- Locations
- United Kingdom
- The impact of the use of Real-World Evidence (RWE) for NICE submissions
Real World Evidence (RWE) refers to scientific evidence generated from observational data collected during routine clinical care. Following the NICE methods review, the latest NICE Health Technology Evaluation Manual formalises the acceptability of RWE as a source of evidence. While in general Randomised Clinical Trials (RCTs) remain the gold standard for clinical evidence, the review goes as far as suggesting some instances where RWE might be considered the preferred source. NICE continues the development of its RWE Framework (a draft version included as Appendix 1 of the manual), which sets out principles and acceptable methodologies for developing RWE.
This formal acceptance of RWE is, however, more a recognition of an existing trend towards RWE than a step-change in policy, with an increasing number of companies using RWE in their regulatory submissions over the past several years. For example, IQVIA research has shown that in rare disease, submissions for approval including RWE are approved faster by most approval pathways than those which don’t 1.
To demonstrate this trend, we utilised IQVIA’s proprietary HTA-Accelerator tool – a codified database of HTAs from multiple jurisdictions – to quantify the use of RWE in submissions to NICE.
Approach
Data from HTA accelerator between 2018-2021 was extracted and HTA records were included for analysis based on the following inclusion criteria:
- HTA body = NICE
- HTA type = Single Drug Assessment
- HTA status = Published
The data set contained 473 fields and for our analysis we identified fields which indicated:
(1) Whether RWE was included as part of the submission
(2) The types of RWE included
(3) The recommendations (positive or negative)
(4) The therapeutic area of the submission
Findings
Overall n=223 submissions were analysed: 56 (2018), 51 (2019), 43 (2020) and 73 (2021).
For 2018, 36% of HTA submissions were identified as having included RWE, whilst in 2021, the corresponding figure was 85%; more than double the proportion.
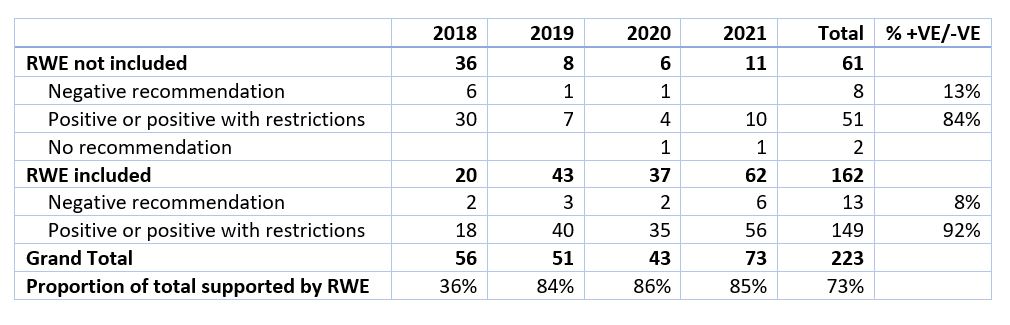
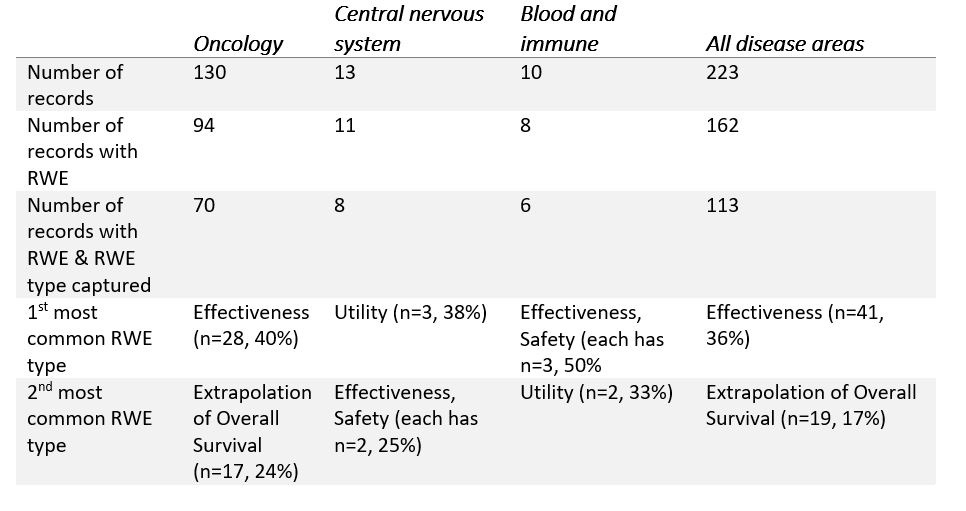
Across the years analysed, 92% of submissions including some form of RWE had positive recommendations, while 84% of submissions excluding RWE had positive recommendations– suggesting that RWE had some role to play in increasing the likelihood of a positive HTA outcome prior to the new manual being issued. As shown in Table 2 below, across all disease areas over 2018-2021, the most common form of RWE submitted was Effectiveness Studies (36%) followed by Extrapolation of Overall Survival (17%).
However, this varied significantly by disease area. For example, RWE pertaining to Extrapolation of Overall Survival (OS) is frequently submitted for Oncology (n=17 from 70 records), but not other therapeutic areas (n=2 out of 43 records). This is because OS is the gold standard outcome measure for Oncology, yet has become increasingly difficult to capture during clinical trials due to improving survival rates and limited follow-up of clinical trial participants after trial closure. Additionally, regulatory approvals are often fast-tracked for new Oncological therapies with less mature OS data due to high unmet needs in target populations, however NICE still require OS data for reimbursement decisions.
Table 2: Most common types of RWE submitted across all therapeutic areas and for the three largest therapeutic areas by number of submissions 2018-2021
Discussion
RWE has been described in the NICE Strategy 2021 - 2026 as an important resource for resolving evidence gaps and allowing patient access to innovations. Whilst the latest NICE manual formalises the use of RWE for technology appraisals, from our analysis we can see RWE was already an important part of most submissions prior to the publication of the new manual, in particular those that achieved a positive recommendation.
There is, however, no “one size fits all” approach to determining the RWE requirements for supporting a submission, and careful thinking is often required for understanding the evidence needs of a specific product given its value proposition, the broader evidence package and HTA body expectations for the therapeutic area.
Whilst RWE is frequently faster and more cost effective to produce than undertaking a clinical trial, there is still a significant lead time. A study utilising an existing database can take a year to complete including ethics approval, and Primary Data Collection studies can take even longer. In our experience, it is important for local and global teams to work together to identify evidence needs well ahead of submissions to NICE.
Interested in finding out more about IQVIA’s thought leadership on RWE? Check out our most recent whitepapers:
- Making Real World Evidence meaningful and actionable: Embedding communication strategies in RWE initiatives
- https://www.iqvia.com/library/white-papers/capturing-value-at-scale-the-$4-billion-rwe-imperative
- Seal of Approval: Accelerating Regulatory success with RWE
- Excellent Launches are winning the evidence battle: Beyond necessity and nice to have: RWE as a true strategic differentiator
1https://www.iqvia.com/library/white-papers/seal-of-approval-accelerating-regulatory-success-with-rwe

