Bring your biosimilar to market faster by tapping into unparalleled data, technology, advanced analytics, and scientific expertise.






















- Insights
- The IQVIA Institute
- Reports and Publications
- Reports
- Assessing the Biosimilar Void
Report Summary
While biosimilar competition in Europe has played a vital role in achieving significant healthcare savings and expanding patient access to key medicines, the changing nature of future loss of exclusivity (LoE) events means that competition, and by extension savings, is not always guaranteed.
This report provides a timely view of the factors underlying the changing level of biologic pipeline activity in Europe, highlighting classes of biologics that are at risk of failing to attract biosimilar competition, a concept called “the biosimilar void.” This report also aims to quantify the potential impact of the biosimilar void on healthcare system budgets. Drawing on a wide range of IQVIA proprietary data and engagement with individual stakeholders, the report examines the cohort of biologic medicines that will lose protection over the next 10 years. The period for assessment (2023–2032) has been chosen to reflect the average development timeline for new biosimilar candidates (~7-10 years) and intrinsic limitations with forecasting data beyond 2032. Due to the evolving nature of the IP landscape in Europe, legal and IP barriers are not discussed in the present study.
Key findings:
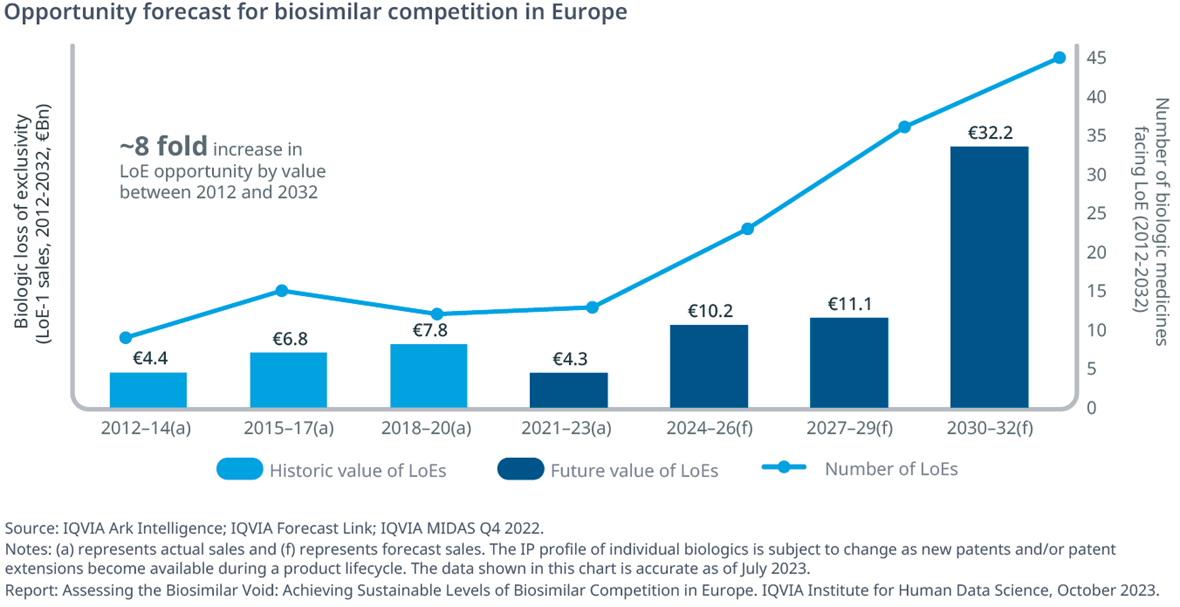
- Between 2021 and 2023, €4.3Bn of biologics have faced off-patent competition (down 45% compared to the previous three years). However, potential savings opportunities are expected to increase rapidly in the short term.
- In Europe, a total of 110 biological medicines are anticipated to lose intellectual property (IP) protection in the next 10 years (by the end of 2032), with LoE opportunities peaking around €30Bn between 2030 and 2032.
- The 8-fold increase in value compared to the 2012-14 period is driven in large part by major LoE events, such as pembrolizumab (Keytruda), daratumumab (Darzalex) and nivolumab (Opdivo). The number and type of biologics losing exclusivity creates unprecedented opportunities for payers, biosimilar developers, and patients.
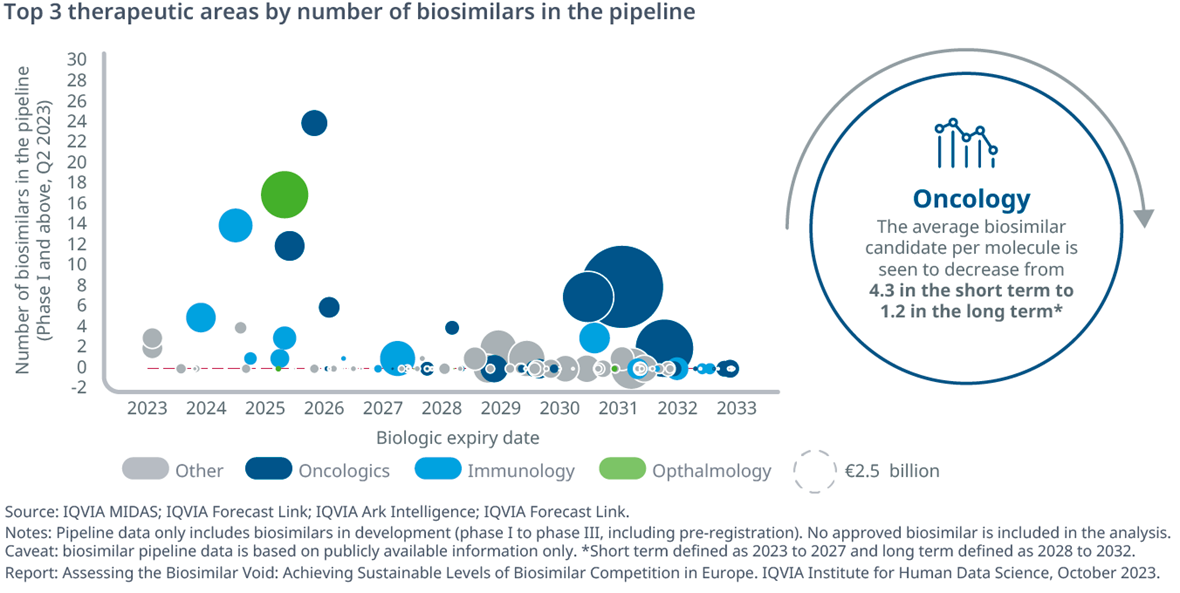
- Over the next 10 years, the majority of biologic LoEs will be oncology biologics (24%), followed by biologics to treat immune system (11%) and blood disorders (10%)
- In the short-term (next five years, by 2027), oncology accounts for the largest share of biosimilar development programs (44%)
- In the long-term (2027 onward), the average number of biosimilars in development is seen to decrease from 2.19 per molecule to 0.43. This trend is driven in large part by a large decrease in the average number of onco-biosimilar candidates, which is seen to decrease from 4.3 (short term, 2023-2027) to 1.2 (long term, 2028–2032)
- The cost and time required to take a biosimilar medicine to market are increasingly constraining the ability of sponsors to develop and launch new products. Critically, these constraints do not only apply to products with a limited commercial opportunity
- The high purchasing costs of the relevant reference comparator biologic products and the large patient samples required to meet the designated clinical endpoints within the current European and global regulatory frameworks are expected to constrain the ability of manufacturers to developing new onco-biosimilar medicines, while ensuring that the market remains commercially viable.
- These factors are expected to decelerate future development activity, increasing the time required for the production and approval of biosimilar medicines in the long term.
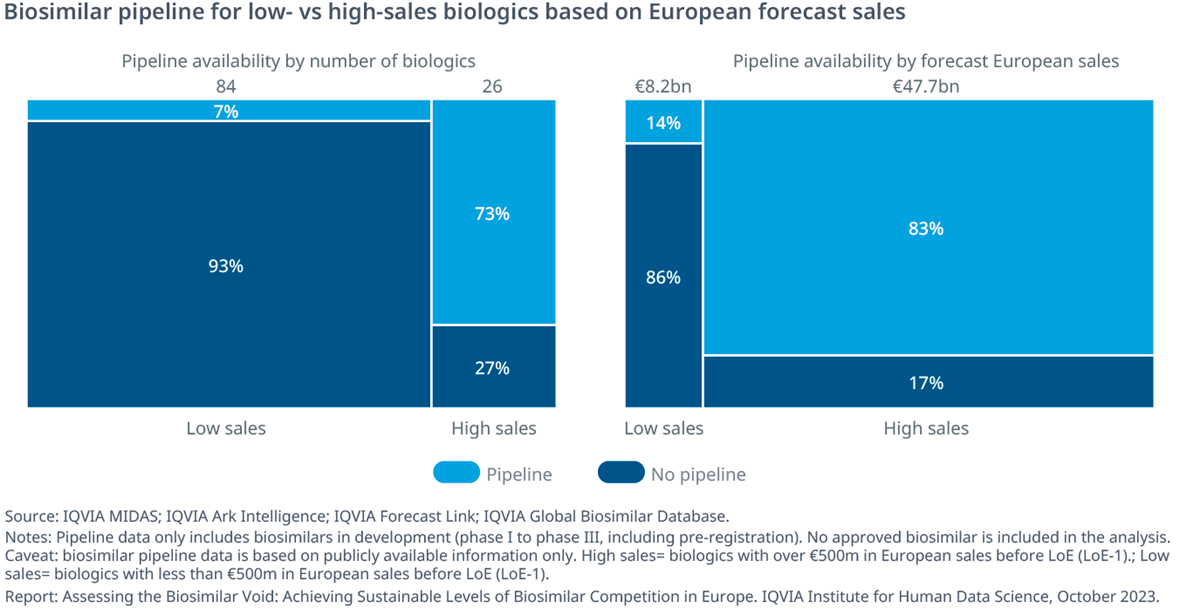
- Of the 26 high-sales products exposed to LoE events in the next 10 years (by end of 2032), almost one in three (27%) does not yet have a biosimilar candidate in the pipeline. This represents ~€8Bn in missed opportunity for payers.
- The large majority of LoE events for biologic medicines in the next 10 years (by end of 2032) are composed of products anticipated to achieve less than €500Mn in annual sales in Europe at the time of expiry (‘low-sales’ category). Due to the limited commercial opportunity, these products are expected to attract low levels of biosimilar development.
- In absolute numbers, 76% (84) of the biologics reaching protection expiry in Europe fit the definition of ‘low-sales.’ Only 7% of them are expected to receive competition in the next 10 years.
- Based on the forecast annual sales value of low-sales products with no biosimilar candidates in the pipeline, the missed opportunity for this segment amounts to ~€7 billion. Therefore, currently available information suggests that the biosimilar void could cost a minimum of ~€15Bn in lost savings, approximately 25% of the total LoE opportunity by 2032.

- Of the total number of medicines with an active orphan medicine status approved in 2022 in Europe, 63% were biologic medicines, up from 53% in 2021.
- Between 2018 and 2022, less than 10% of all biologics reaching protection expiry in Europe carried an orphan designation. In the next 10 years (by end of 2032), the share of orphan biologics losing exclusivity is expected to reach 34% as innovators continue to invest in orphan medicines.
- Despite the fast-growing opportunity for orphan biosimilar medicines, very few are in development. Only one orphan biologic (eculizumab) has so far attracted biosimilar development, corresponding to less than 3% of the entire cohort.
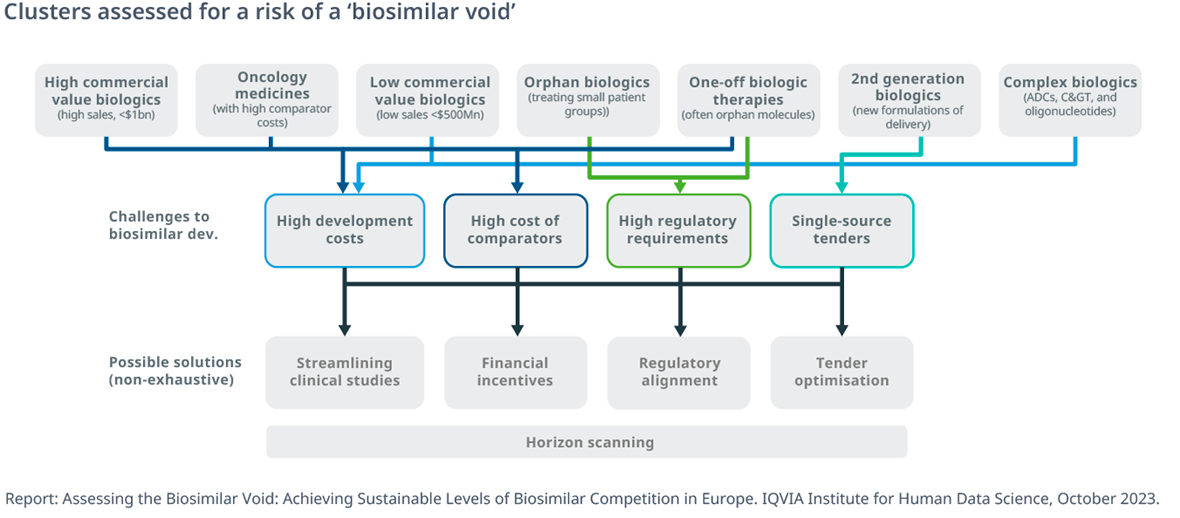
- As the number of biologic medicines losing exclusivity continues to increase, achieving sustainable levels of off-patent competition will require a careful rebalancing and consideration
- With fewer entrants into the market, competition between the originators and biosimilar medicines could resemble competition between brand-name medicines, with fewer products, and a reduction in price discounts.
- Failure to address the barriers to the challenges of biosimilar development is likely to increase the financial burden of European healthcare systems while reducing their impact to improve patient access. This report shows a complex set of challenges that limit the availability of biosimilar medicines in Europe in the coming years, leading to a missed opportunity of at least ~€15Bn in cost savings, with significant implications for biologic treatment cost efficiency gains and average increase in the number of eligible patients treated.
- A detailed assessment of clusters of biologics at risk of limited or no competition highlights areas for investigation and improvement for global and EU stakeholders.
Related solutions
Specialized expertise and customized solutions across 14 therapeutic centers of excellence, including oncology, GI/NASH, pediatrics, neurology and rare diseases.





